About Us
Your Trusted Medical Supply Partner
Incorporated in 2018, Tamaki Group is proud to be New Zealand's first 100% Māori owned medical supply company.
For us, being kaupapa Māori means living through tikanga – embracing Māori culture and values inclusive of all people and ethnicities.
He Taru Tawhiti (A weed from far away) is a metaphor for the unfamiliar diseases that arrived with the early settlers and aptly describes the many viruses and pathogens that threaten the health and wellbeing of our whanau and communities today.
Tamaki Group's products serve to protect our community from these risks, underpinned by Māori values, protocols, concepts, views of health and Te Tiriti o Waitangi
We supply governments, healthcare providers, services and institutions with high-quality medical PPE, delivered fast, at fair pricing.
Face Mask FAQs
What are the different types of single use face masks?
Single use face masks, also referred to as surgical masks, provide a protective barrier that prevents the spread of larger droplets from the wearer to others, as well as protecting the wearer from inhaling larger droplets, and from fluid splashes or high velocity streams of bodily fluids.
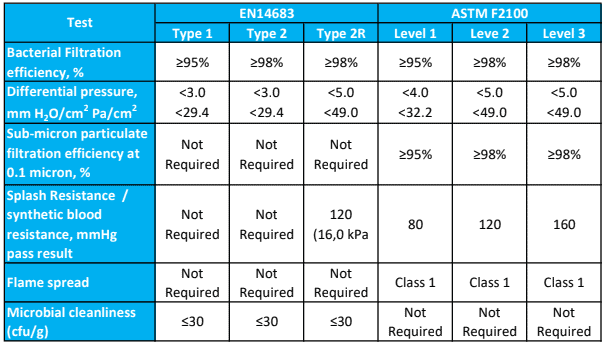
Single use face masks are rated as Level 1, Level 2 or Level 3 according to the degree of barrier protection provided.
Single use face mask: barrier protection and COVID-19 application:
Barrier Level 1
For procedures where the wearer is not at risk of splash or spray from blood or bodily substances, or to protect staff and/or patients from droplet exposure to microorganisms.
COVID-19 application: Tier 1 – Area of higher clinical risk and where the patient is NOT suspected or confirmed to have COVID-19 and is NOT in quarantine
Barrier Level 2
Where minimal blood droplet exposures may occur, such as changing dressings on small wounds or healing wounds.
COVID-19 application: Tier 2 – Droplet and contact precautions. Direct care or contact with a person who is suspected or confirmed to have COVID-19 or is in quarantine.
Barrier Level 3
All surgical procedures, major trauma first aid or whenever there is a risk of blood or bodily fluid splash or spray, such as orthopaedic or cardiovascular procedures.
COVID-19 application: Tier 2 – Droplet and contact precautions. Direct care or contact with a person who is suspected or confirmed to have COVID-19 or is in quarantine.
What is a Type IIR Face Mask?
Type IIR face masks EN14683 are medical face masks made up of a 3 ply construction that prevents large particles from reaching the patient or working surfaces.
Type IIR Face masks include a splash resistant layer to protect against blood and other bodily fluids. Type IIR face masks are tested in the direction of exhalation (inside to outside) and take into account the efficiency of bacterial filtration.
Characteristics of Type IIR face masks include:
Pleat style with ear loops or ties
Protective four-layer construction
Available in a variety of colours and styles
Splash resistant layer against bodily fluids.
Type I, Type IR, Type II and Type IIR masks are for use in protecting others from the wearer transmitting infection.
How do I work out what level mask I have?
How many layers do the masks have?
Our masks have 3 layers:
Inner layer: PP non-woven
Middle layer: Melt-blown filter
Outer layer: PP non-woven
What is a melt-blown filter?
Melt blowing is a conventional fabrication method of micro- and nanofibers, where a polymer melt is extruded through small nozzles, while surrounded by high-speed blowing gas. The randomly deposited fibres form a non-woven sheet product, applicable for filtration.
What is BFE?
BFE stands for Bacterial Filtration Efficiency.
Our masks are tested to a BFE of >98%, meaning that the possibility of contracting a virus is significantly reduced.
What is BFR or Blood Fluid Resistance?
Testing for fluid resistance against a high-pressure jet of simulated blood aims to replicate an arterial puncture which is sprayed directly at the face mask or respirator. The parameters for testing are based on the mean human blood pressure (80 – 120 mmHg) and likely proximity to the puncture site.
The American Society for Testing and Materials (ASTM) F1862 / F1862M – 17 classification system rates a face mask or respirator as Level 1, Level 2 or Level 3 based on its resistance to a 2ml high velocity directed blood spray at a distance of 300mm:
Level 1: resistant at 80mmHg
Level 2: resistant at 120mmHg
Level 3: resistant at 160mmHg